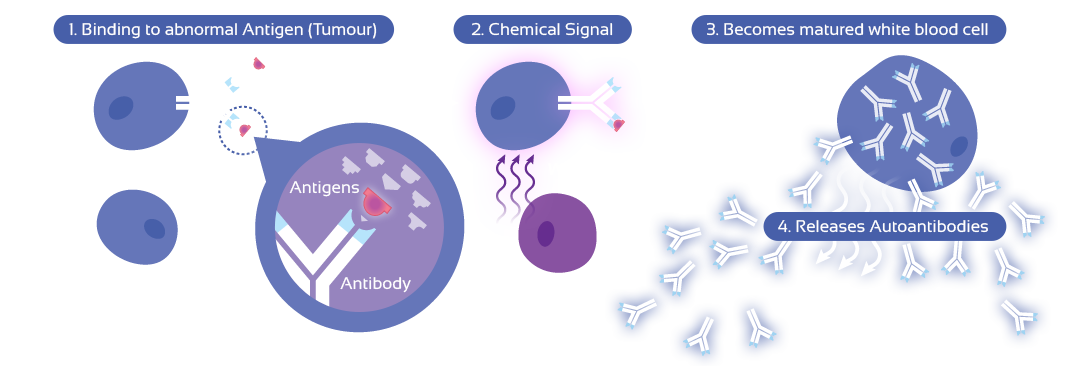
Why autoantibodies work better?
Traditional check-ups for lung diseases are often performed through imaging techniques such as X-ray. Small shadows may be missed or sometimes ignored, as there is a possibility of benign nodules or scars from other diseases. Treatment could be delayed due to the oversight of the imaging result especially during the early stages of cancer. Thus after several years, the tumour might only be diagnosed in the latter stages of growth and this becomes more difficult to treat. With the assistance of autoantibodies however, the story can be different.

When a tumour is present in the body, it releases some of its proteins (known as antigens) into the blood stream - these antigens are not normally found in the body. The body’s immune system then reacts to these abnormal antigens by producing autoantibodies in an attempt to fight this abnormality.

Since a small amount of cancerous antigen molecules can trigger the body to release the autoantibodies, this immune response acts as a very efficient biological amplification system as it boosts the autoantibodies’ level up to a measurable amount. By detecting autoantibodies in the blood instead of antigens, the presence of a tumour can be detected at a much earlier stage and a more specific prognosis can be drawn up.
Finally, the persistency and stability of autoantibodies in the blood is another advantage to those protein markers that are released directly by tumours as they are being rapidly broken down or cleared out after circulating in the blood.
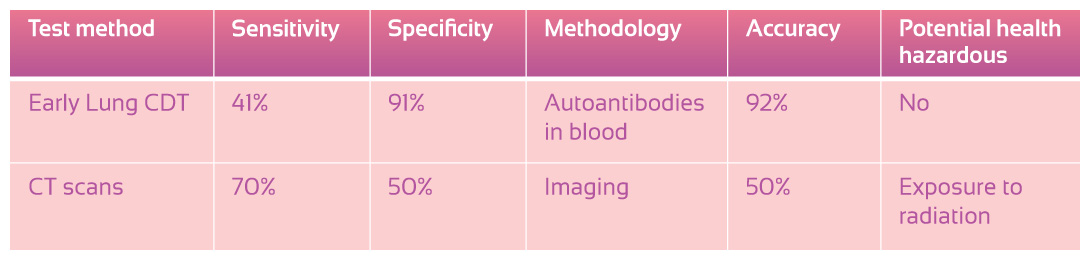
Currently, the main methods of lung cancer detection include X-ray and CT (Computed tomography) scan, but these involve some levels of radiation exposure and can prove harmful if repeated imaging is needed. Testing for the presence of autoantibodies within the blood does not carry this risk.
What is EarlyCDT-Lung intended to do?
The goal of this autoantibodies lung cancer test is to detect lung cancer early. Currently most lung cancer cases are only detected once symptoms appear and usually in later stages of the disease. By testing against a validated panel of seven autoantibodies, the possibility of detecting cancer development early is greatly increased since the test is specifically designed to indicate the presence of cancerous cells in the body at any stage and with high accuracy. Since the body automatically produces autoantibodies before any other detectable symptoms develop as a result of lung cancer, it literally pre-empts the disease.
The selected and validated autoantibodies for EarlyCDT-Lung are:






Reference support
Chapman C. J. et al. EarlyCDT-Lung test: Improved clinical utility through additional autoantibody assays. Tumor Bio. 2012; 1319 – 1326.
Oken MM et al. Screening by Chest Radiograph and Lung Cancer Mortality. JAMA 2011; 306(17); 1865 – 73.
Desmetz C. et al Identifying autoantibody signatures in cancer: a promising challenge. Expert Rev Proteomics. 2009; 377 – 86.
Murray A. et al. Technical validation of an autoantibody test for lung cancer. Annals of Oncology. 2010; 1687–1693.
Lu H. et al Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J. Proteome Res. 2007; 1388 – 1394.
Swensen SJ et al, CT screening for lung cancer: Five year prospective experience. Radiology 2005; 235; 259 –265.